When taking any kind of medication, there’s one thing that consumers always assume is true: Each pill in their prescription is formulated with the exact ingredients promised. However, pharmaceutical company Allergan is now voluntarily recalling nearly 170,000 packs of its birth control pills in the U.S. because a few of their pills were not what they were promised to be. In fact, a packaging error put placebo pills where hormonal pills were supposed to be, increasing the risk of unintended pregnancy in users.
According to a statement announcing the recall, Allergan explains that thousands of Taytulla birth control packs had four placebo pills placed out of order, putting four non-hormonal pills within the first four days of treatment instead of the active pills. So, anyone starting the affected pack would unknowingly be forgoing the active pills they need to prevent pregnancy.
You May Also Like: Amazon’s Big Beauty Problem
Luckily, it’s easy to check which birth control packs are ineffective. Allergan says that the lot number 5620706, expiring in May 2019, are the only packs that have been incorrectly packaged. The brand also provided the below image to distinguish which packs should be disregarded:
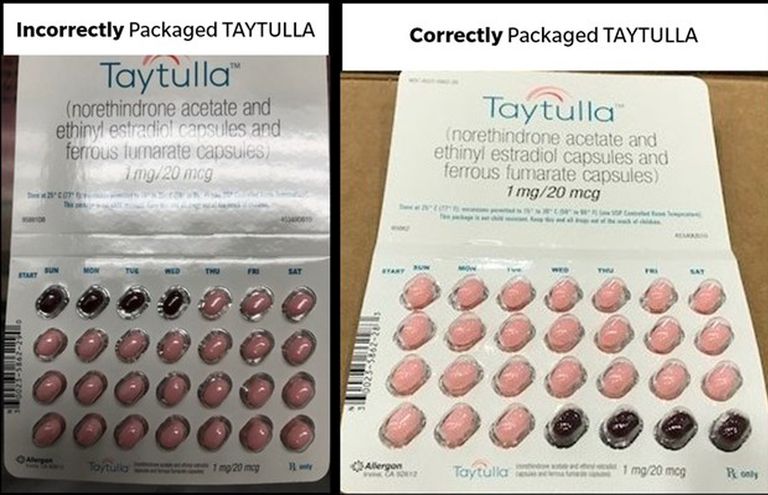
Image/Allergan
Basically, if the first four pills of the pack being used are dark red and the rest are light pink, your pill pack is part of the recall. Anyone with a birth control pack that is included in the recall should consult their doctor, and for those with additional questions regarding their Taytulla birth control, Allergan can be reached at 800-678-1605.